1. Fats generally are solidwhile the
oil is liquid phase, however both are equally triglyceride. Explain why the
from of two . Different triglyceride, and point out important factors that
determine the from of fat.
Answer : fat is saturated and oil is
unsaturated and then fat have high van derwalls and oil have low van derwalls
so that fat are solid phase in room temperature and oil are liquid phase in
temperature.
A fat's constituent fatty acids may
also differ in the C/H ratio. When all three fatty acids have the formula CnH(2n+1)CO2H,
the resulting fat is called "saturated". Values of n usually
range from 13 to 17. Each carbon atom in the chain is saturated with hydrogen,
meaning they are bonded to as many hydrogens as possible. Unsaturated
fats are derived from fatty acids with the formula CnH(2n-1)CO2H.
These fatty acids contain double bonds within carbon chain. This
results in an "unsaturated" fatty acid. More specifically, it would
be a monounsaturated fatty acid. Polyunsaturated fatty
acids would be fatty acids with more than one double bond; they have the
formula, CnH(2n-3)CO2H and CnH(2n-5)CO2H.
Unsaturated fats can be converted to saturated ones by the process
of hydrogenation. This technology underpinned the development
of margerine.
Saturated and unsaturated fats
differ in their energy content and melting point. Since unsaturated fats
contain fewer carbon-hydrogen bonds than saturated fats with the same number of
carbon atoms, unsaturated fats will yield slightly less energy during
metabolism than saturated fats with the same number of carbon atoms. Saturated
fats can stack themselves in a closely packed arrangement, so they can freeze
easily and are typically solid at room temperature. For example, animal
fats tallow and lard are high in saturated fatty acid
content and are solids. Olive and linseed oils on the other hand are highly
unsaturated and are oily.
2. How primary metabolite can be
converted into secondary metabolisme. what is the basic idea and how the
mechanism could be desribed.
Answer: basic ideas to convered
primary metabolite into secondary metabolite is from the reaction the
fundamental processes of photosynthesis, glycolysis, and the Krebs cycle are
tapped off from energy-generating processes to provide biosynthetic
intermediates.To make biosynthesis intermediets needs the buillding blocks. By
far the most important building blocks employed in the biosynthesis of
secondary metabolites are derived from the intermediates acetyl coenzyme A
(acetyl-CoA), shikimic acid, mevalonic acid, and methylerythritol phosphate.
These are utilized respectively in the acetate, shikimate,
mevalonate, and methylerythritol phosphate pathways, Acetyl-CoA is
formed by oxidative decarboxylation of the glycolytic pathway product pyruvic
acid. It is also produced by the β-oxidation of fatty acids, effectively
reversing the process by which fatty acids are themselves synthesized from
acetyl-CoA. Important secondary metabolites formed from the acetate pathway
include phenols, prostaglandins, and macrolide antibiotics, together with
various fatty acids and derivatives at the primary–secondary metabolism
interface. Shikimic acid is produced from a combination of
phosphoenolpyruvate, a glycolytic pathway intermediate, and erythrose
4-phosphate from the pentose phosphate pathway. The reactions of the pentose
phosphate cycle may be employed for the degradation of glucose, but they also
feature in the synthesis of sugars by photosynthesis. The shikimate pathway
leads to a variety of phenols, cinnamic acid derivatives, lignans, and
alkaloids. Mevalonic acid is itself formed from three molecules of
acetyl-CoA, but the mevalonate pathway channels acetate into a different series
of compounds than does the acetate pathway. Methylerythritol phosphate arises
from a combination of two glycolytic pathway intermediates, namely pyruvic acid
and glyceraldehyde 3-phosphate by way of deoxyxylulose phosphate. The
mevalonate and methylerythritol phosphate pathways are together responsible for
the biosynthesis of a vast array of terpenoid and steroid metabolites.
In addition to acetyl-CoA, shikimic acid, mevalonic acid, and
methylerythritol phosphate, other building blocks based on amino acids are
frequently employed in natural product synthesis. Peptides, proteins,
alkaloids, and many antibiotics are derived from amino acids, and the origins
of some of the more important amino acid components of these are briefly
indicated in Figure 2.1. Intermediates from the glycolytic pathway and the
Krebs cycle are used in constructing many of them, but the aromatic amino acids phenylalanine,
tyrosine, and tryptophan are themselves products from the
shikimate pathway. Ornithine, an amino acid not found in proteins, and its
homologue lysine, are important alkaloid precursors and have their origins
in Krebs cycle intermediates. Of special significance is the appreciation that
secondary metabolites can be synthesized by combining several building blocks
of the same type, or by using a mixture of different building blocks. This
expands structural diversity and, consequently, makes subdivisions based
entirely on biosynthetic pathways rather more difficult. A typical natural
product might be produced by combining elements from the acetate, shikimate,
and methylerythritol phosphate pathways.
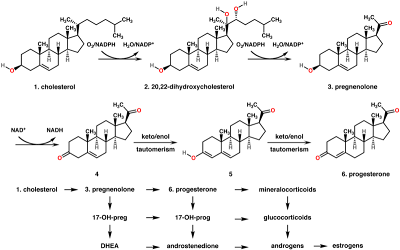
3. Hormone progesterone is essensial for the survival of
the pregnancy. these hormones are derived from a steroid biogenetically.
explain the logic of chemical reactions which may occour in the formation progesterone.
answer :
Biosynthesis
in
mammals progesterone, like all other steroid hormones, is
synthesized frompregnenolone, which in turn is derived from cholesterol.
Cholesterol undergoes double
oxidation to produce 20,22-dihydroxycholesterol. This vicinal diol is then further
oxidized with loss of the side chain starting at position C-22 to produce
pregnenolone . This reaction is catalyzed bycytochrome P450scc. The
conversion of pregnenolone to progesterone takes place in two steps. First, the
3-hydroxyl group is oxidized to a keto group and second,
the double bond is moved to C-4, from C-5 through a keto/enol tautomerizationreaction. This
reaction is catalyzed by 3beta-hydroxysteroid dehydrogenase/delta -delta
isomerase.
Progesterone in trun is the
precursor of the mineralocorticoid aldosterone, and after conversion
to 17-hydroxyprogesterone(another natural progestogen) of cortisol and androstenedione.
Androstenedione can be converted
to testosterone, estrone and estradiol.
Top: Conversion of cholesterol (1) into pregnenolone (3) to progesterone (6).
Bottom: Progesterone is important for aldosterone (mineralocorticoid) synthesis, as 17-hydroxyprogesterone is for cortisol (glucocorticoid), and androstenedione for sex steroids.
In laboratory
An economical semisynthesis of
progesterone from the plant steroid diosgenin isolated from yams was
developed by Russell Marker in 1940 for the Parke-Davis pharmaceutical
company (This synthesis is known as the Marker degradation. Additional
semisyntheses of progesterone have also been reported starting from a variety
of steroids. For the example, cortisone can be simultaneously
deoxygenated at the C-17 and C-21 position by treatment with
iodotrimethylsilane inchloroform to produce 11-keto-progesterone
(ketogestin), which in turn can be reduced at position-11 to yield
progesterone. Pregenolone and progesterone can also be synthesized by
yeast.
The
Marker semisynthesis of progesterone fromdiosgenin
A total
synthesis of progesterone was reported in 1971 by W.S.
Johnson. The synthesis begins with reacting the phosphonium
salt 7 with phenyl lithium to produce
the phosphonium ylide 8 .
The ylide is reacted with analdehyde to produce the alkene 9.
The ketal protecting groups of 9 are
hydrolyzed to produce the diketone 10, which in turn is
cyclized to form the cyclopentenone 11. The ketone of 11 is
reacted with methyl lithium to yield the tertiary alcohol 12,
which in turn is treated with acid to produce the tertiary cation 13.
The key step of the synthesis is the π-cation cyclization of 13 in
which the B-, C-, and D-rings of the steroid are simultaneously formed to
produce 14. This step resembles the cationic cyclization
reaction used in the biosynthesis of steroids and hence is referred to as biomimetic.
In the next step the enolorthoester is hydrolyzed to produce the ketone 15.
The cyclopentene A-ring is then opened by oxidizing with ozone to produce16.
Finally, the diketone 17 undergoes an
intramolecular aldol condensation by treating with aqueous potassium
hydroxide to produce progesterone.
The
Johnson total synthesis of progesterone
4. Many alkaloid are
toxic to other organisms. They often have pharmacological effects and are used
as medications, as recreational drugs, or in entheo genic rituals. Desribe in
outline the process of biosynthesus of an alkaloid compound and desribe the function
groups which play an important role in the biological activities.
Answer :
biosynthesis of alkaloid compound example from purine alkaloid. The
purine derivatives caffeine, theobromine, and theophylline are usually referred
to as purine alkaloids. They have a rather limited distribution, and
their origins are very closely linked with those of the purine bases adenine and
guanine, fundamental components of nucleosides,
nucleotides, and the nucleic acids. Caffeine, in the form of
beverages such as tea, coffee, and cola, is one of the most widely
consumed and socially accepted natural stimulants. It is also used medicinally,
but theophylline is more important as a drug compound because of its muscle relaxant
properties, utilized in the relief of bronchial asthma.
Theobromine is a major constituent of cocoa and related
chocolate products . The purine ring is gradually elaborated by piecing together small
components from primary metabolism. The largest component
incorporated is glycine, which provides a C2N unit, whilst the remaining carbon atoms come
from formate and bicarbonate. Two of the four nitrogen atoms are supplied
by glutamine and a third by aspartic acid. Synthesis of the nucleotides
adenosine 5_-monophosphate (AMP) and guanosine 5_-monophosphate (GMP) is by way
of inosine 5_-monophosphate (IMP) and xanthosine 5_-monophosphate (XMP) (Figure
6.141), and the purine alkaloids then branch away through XMP. AMP, if
available, can also serve as a source of IMP. Methylation and then loss of phosphate
generates the nucleoside 7-methylxanthosine, which is then released from the sugar. Successive
methylations on the nitrogen atoms give caffeine by way of theobromine, whilst a different methylation sequence can account for
the formation of theophylline. Theophylline can
also be produced by demethylation of caffeine as part of a degradative pathway.
Some of the N-methyltransferases
display rather broad substrate specificity, and this allows minor pathways to
operate in certain plants, e.g. the alternative sequence to 7-methylxanthosine
via 7-methyl XMP shown in Figure 6.141. In addition, the enzyme caffeine
synthase in coffee (Coffea arabica; Rubiaceae) has dual functionality,
and methylates both theobromine and 7-methylxanthine; a tea (Camellia
sinensis; Theaceae) enzyme is specific for theobromine.
This is purine synthesis
This is biosynthesis purine alakaloid to produced caffeine,
theobromine, and theophylline





Tidak ada komentar:
Posting Komentar